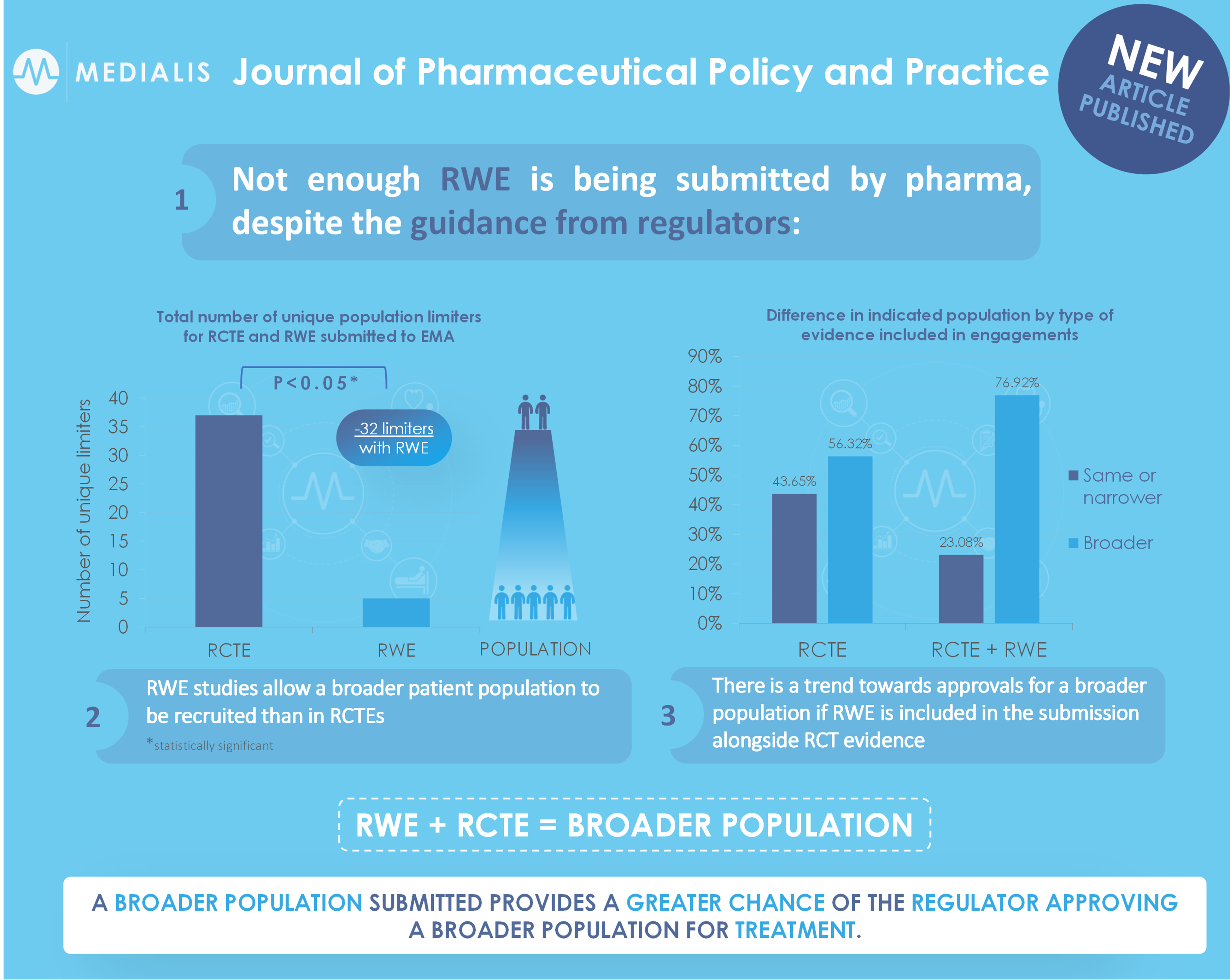
The effect of adding real-world evidence to regulatory submissions on the breadth of population indicated for rare disease medicine treatment by the European Medicines Agency
Background In 2020, the European Medicines Agency (EMA) published EMA Regulatory Science to 2025, which included among its top five core recommendations the promotion of the use of high-quality real-world data in regulatory decision making [1]. The OPTIMAL framework is a set of criteria developed by the EMA for the regulatory use of RWE, which...