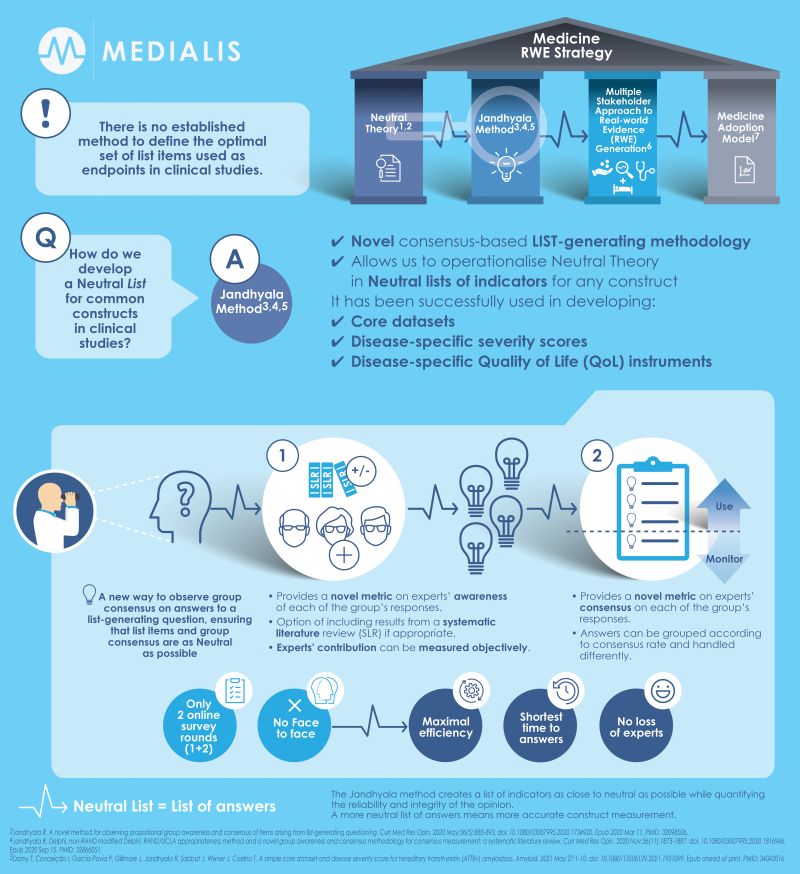
Medialis Ltd Develops First Long-COVID Disease-Specific Quality of Life Instrument: PAC-19QoL
In early 2020, as the COVID-19 pandemic reshaped our world, Medialis Ltd recognised the urgent need for a disease-specific quality of life (QoL) instrument for long COVID patients. This, led to the development of the PAC-19QoL. Rapid Development Timeline: We received ethical approval in October 2020 and initiated the development of the PAC-19QoL on November...